Enhancing Pharmaceutical Quality Control: Importance of Gelatin Capsule Hardness Testing
Discover why gelatin capsule hardness testing, including softgels, is crucial in pharmaceutical manufacturing. Learn about the significance of the Cell Instruments CHT-01 capsule hardness tester for ensuring product integrity, dosage accuracy, and regulatory compliance.
Testing the hardness of gelatin capsules, including both hard capsules and softgels, is an indispensable aspect of pharmaceutical manufacturing and quality control processes.
This comprehensive examination ensures that the capsules meet stringent standards of robustness and integrity, guaranteeing the efficacy, safety, and reliability of the encapsulated medications. In this extensive discussion, we delve into the significance of capsule hardness testing, its implications for product quality and regulatory compliance, and the pivotal role of advanced testing equipment such as the Cell Instruments CHT-01 capsule hardness tester.
Gelatin capsules, widely used in the pharmaceutical industry for encapsulating medications, come in two primary forms: hard capsules and softgels.
Both variants serve as convenient and efficient dosage forms for administering a wide range of drugs, including oral medications, dietary supplements, and over-the-counter pharmaceuticals. However, the physical properties of gelatin capsules, particularly their hardness, play a crucial role in ensuring the overall quality and performance of the final product.
Hardness testing of gelatin capsules involves measuring the resistance of the capsule shell to deformation or breakage under applied pressure.
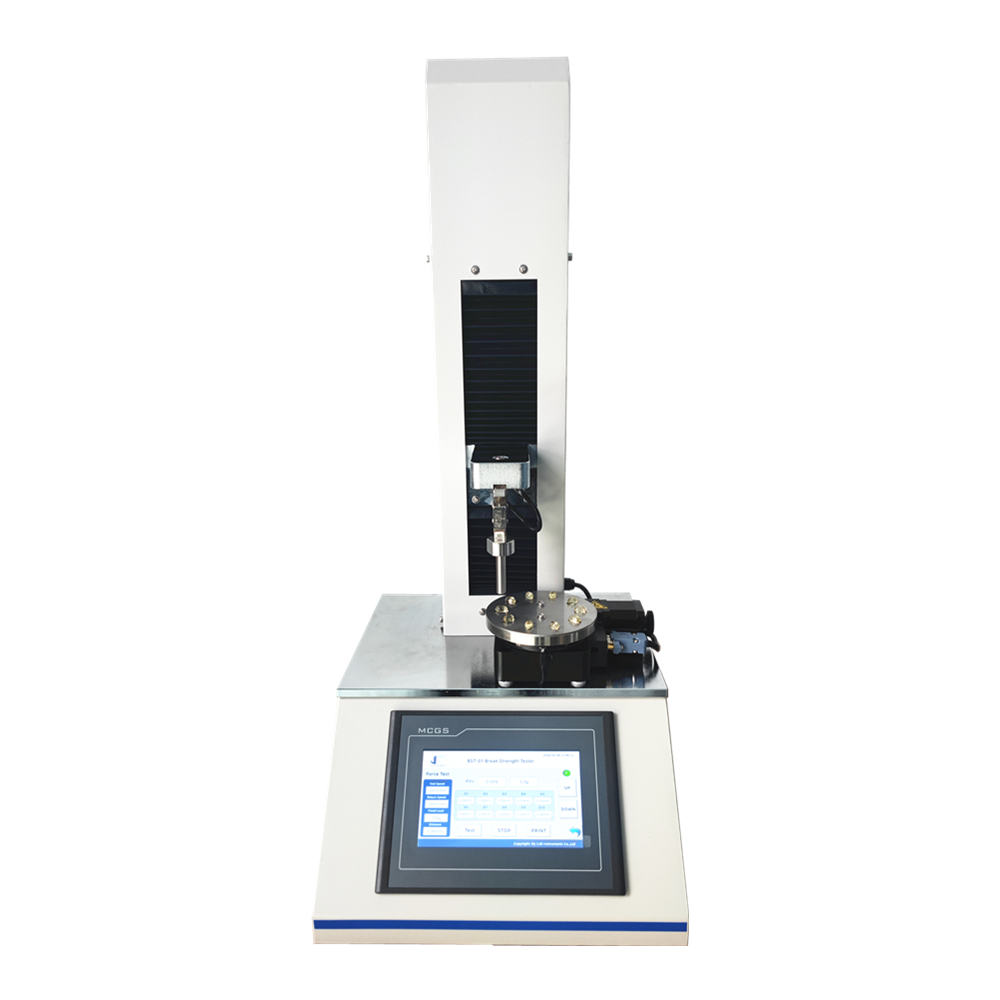
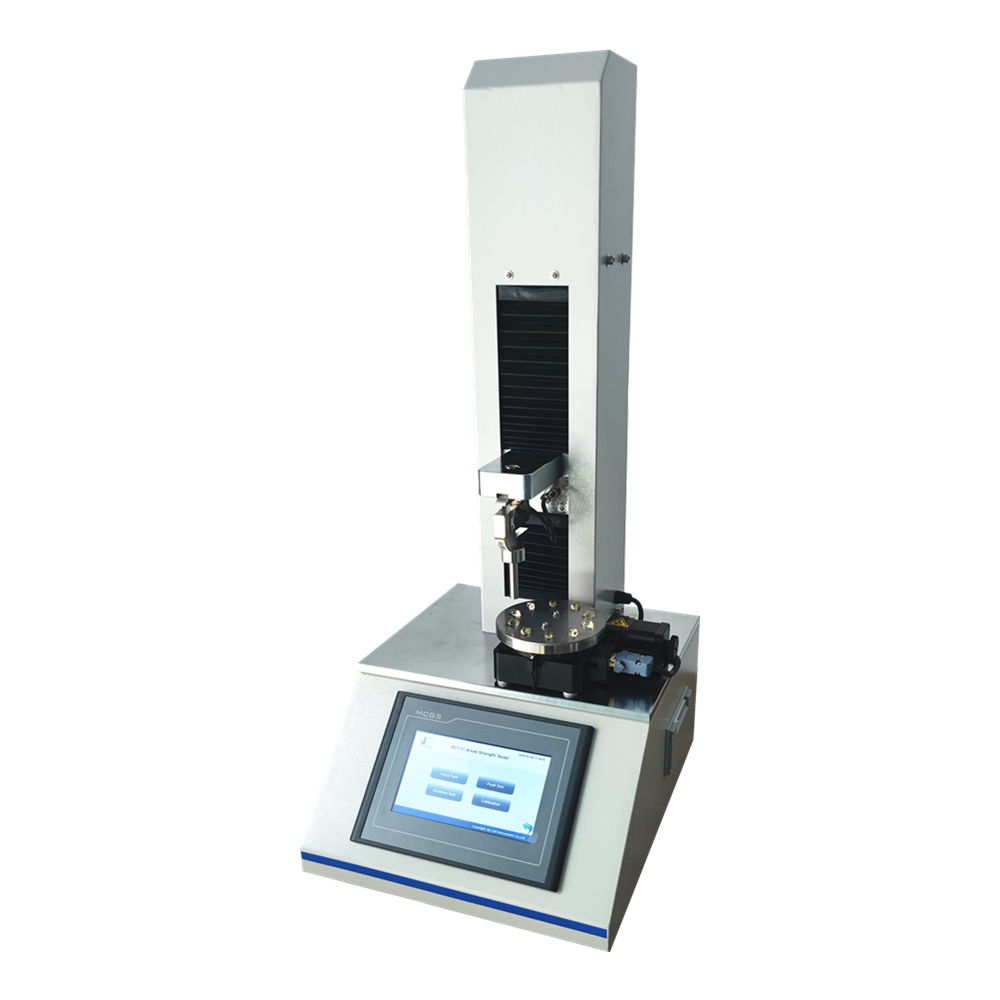
This parameter is typically assessed using specialized equipment, such as the Cell Instruments CHT-01 capsule hardness tester, which applies controlled force to the capsules and records the resulting deformation or disintegration. By quantifying the hardness of capsules, manufacturers can ascertain their mechanical strength and durability, which are essential for withstanding the rigors of manufacturing processes, transportation, storage, and handling by consumers.
The importance of capsule hardness testing extends beyond mere quality assurance; it encompasses several critical aspects of pharmaceutical manufacturing and product development:
Quality Assurance and Product Integrity: Capsule hardness directly influences the overall quality and integrity of pharmaceutical products. Capsules with inadequate hardness may be prone to breakage or leakage, compromising the stability and efficacy of the encapsulated medications. By conducting rigorous hardness testing, manufacturers can identify and rectify any issues related to capsule strength, ensuring that the final products meet established quality standards and regulatory requirements.
Dosage Uniformity and Accuracy: Consistent capsule hardness is essential for achieving uniformity and accuracy in medication dosing. Capsules that are too soft or too hard may exhibit variability in drug release kinetics, leading to inconsistent therapeutic outcomes and potential risks to patient safety. By maintaining optimal hardness levels, manufacturers can ensure that each capsule delivers the intended dose of medication reliably and predictably.
Patient Acceptance and Compliance: The physical properties of gelatin capsules, including their hardness, significantly impact patient acceptance and compliance with medication regimens. Capsules that are easy to swallow, handle, and dispense enhance the overall patient experience, fostering greater adherence to prescribed treatments. Conversely, capsules with suboptimal hardness characteristics may deter patients from using the medication as directed, potentially compromising therapeutic outcomes and disease management.
Regulatory Compliance and Good Manufacturing Practices (GMP): Regulatory agencies, such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe, mandate stringent requirements for pharmaceutical manufacturing, including the testing of capsule hardness. Compliance with these regulations is essential for obtaining market approval and maintaining licensure for pharmaceutical products. By adhering to good manufacturing practices (GMP) and conducting comprehensive hardness testing, manufacturers demonstrate their commitment to quality, safety, and regulatory compliance.
Optimization of Manufacturing Processes: Hardness testing serves as a valuable tool for optimizing manufacturing processes and formulations. By monitoring and controlling capsule hardness throughout various stages of production, manufacturers can identify potential issues, such as formulation inconsistencies or equipment malfunctions, and implement corrective measures promptly. This proactive approach not only improves product quality but also enhances production efficiency and cost-effectiveness in the long run.
In recent years, technological advancements have revolutionized capsule hardness testing, enabling manufacturers to achieve greater precision, accuracy, and efficiency in their quality control processes. The Cell Instruments CHT-01 capsule hardness tester exemplifies this trend, offering advanced features and capabilities for evaluating the mechanical properties of gelatin capsules with unparalleled reliability and reproducibility. Equipped with state-of-the-art sensors, software algorithms, and user-friendly interfaces, this cutting-edge instrument enables pharmaceutical companies to streamline their hardness testing procedures and obtain precise measurements with minimal effort.
The Cell Instruments CHT-01 capsule hardness tester employs innovative testing methodologies, including compression testing and penetration testing, to assess the hardness of gelatin capsules comprehensively. During compression testing, the instrument applies controlled axial pressure to the capsules, simulating the forces exerted during manufacturing, packaging, and transportation. By measuring the force-displacement relationship, the tester determines the hardness profile of the capsules and identifies any deviations from the desired specifications.
In addition to compression testing, the Cell Instruments CHT-01 capsule hardness tester offers penetration testing capabilities, allowing for the precise measurement of capsule shell thickness and resistance to puncture. This feature is particularly useful for evaluating the mechanical properties of softgel capsules, which have distinct characteristics compared to traditional hard capsules. By quantifying the puncture resistance of softgel capsules, the tester provides valuable insights into their structural integrity and performance under different environmental conditions.
Moreover, the Cell Instruments CHT-01 capsule hardness tester integrates seamlessly with other quality control systems and laboratory equipment, facilitating data management, analysis, and reporting. Its intuitive software interface allows users to customize testing parameters, and generate comprehensive test reports. The tester enables seamless integration into existing manufacturing workflows and quality management systems.
In conclusion, capsule hardness testing is a critical aspect of pharmaceutical manufacturing and quality control processes, ensuring the integrity, efficacy, and safety of gelatin capsules used for drug delivery. By utilizing advanced testing equipment such as the Cell Instruments CHT-01 capsule hardness tester, manufacturers can optimize their quality control procedures, comply with regulatory requirements, and deliver high-quality medications that meet the needs and expectations of healthcare providers and patients alike. With its innovative features, precision measurement capabilities, and user-friendly interface, the CHT-01 tester represents a significant advancement in capsule hardness testing technology, empowering pharmaceutical companies to achieve excellence in product quality and performance.
Comments
Post a Comment